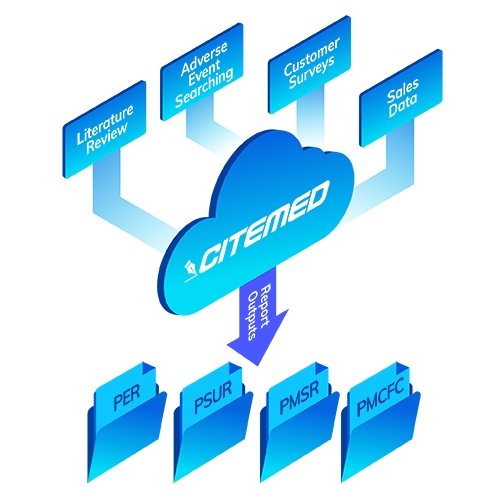
Comprehensive Reporting


The Only Post Market Surveillance Decision You Need to Make
Post Market Surveillance under MDR has become a pain. Believe us, we understand. With multiple documents to juggle, different submission intervals, and (*cough*) ambiguity in the regs, it’s hard to stay organized.
Our team has built the experience, tools and processes to handle it all, year after year. All you have to do let us use them.

EU MDR Submission Expertise
We’ve been in the audit trenches, and aside from a scar or two have lived to submit another day. If we’re handling your submissions, you can rest assured that we’re operating on a breadth of collective experience.
Industry Leading Tools
Your data is too precious and complex to be managed through Excel sheets renamed ‘old’ as a means for version control. Without a system, it’s impossible to maintain a single device document, let alone hundreds of device documents.
That’s why we’ve invested years, and our children’s college funds (just kidding) in building State of the Art tools to keep your Adverse Events, Scientific Literature, and Documents managed.
FEATURED IN


FEATURED IN

Our Clients Say
Free Articles and Post Market Surveillance Whitepapers
How Risk Management and Post-Market Surveillance Interact with Clinical Evaluation
Developing a medical device is not a one-step process. There are hundreds of little steps, from drawing the design to finalizing it to actually creating the device. Between all these steps, there is always [...]
Post-market Clinical Follow-up (PMCF): What you Need and How to Get It
One of the new aspects introduced in the European Medical Device Regulation 2017/745 (MDR) is post-market clinical follow-up (PMCF). While PMCF is well-known in the pharmaceutical world, it is a relatively new concept in [...]
PMCF for IVDR and MDR – A Case Study and How-To
By now, most of us have struggled through at least one successful submission for MDR/IVDR, and we know they take time. Very doable, but no ones exactly surprised at home simple everything was to [...]
Post-market surveillance under the IVDR: Frequently Asked Questions
Requirements for post-market surveillance (among a host of other requirements for everything from classification to clinical follow-up) were never that elaborate in the In Vitro Diagnostics Directive (IVDD; 98/79/EC). However, with the introduction of [...]
Your Medical Device Got CE Marked – Now What?
Obtaining the CE mark is one of the most significant milestones in the life of most medical devices. Unfortunately, it typically takes years and can exhaust even the most determined regulatory personnel. Nevertheless, getting [...]
Market Surveillance of Medical Devices Under the EU MDR
The term market surveillance covers the activities carried out by national regulatory authorities to ensure the safety and efficiency of products placed on the market. In these activities, the national regulatory authorities put measures [...]
Worry-Free EU MDR Compliance

1. Searching and Data Ingress
CiteMed (Through Automation and Careful Human Auditing) Collects Adverse Event Data from all of your chosen global sources
2. Expert Review of All Search Results
Our team of medical writers reviews the results and classifies any relevant Events for you to be included in the next Vigilance Report. (this is optional).

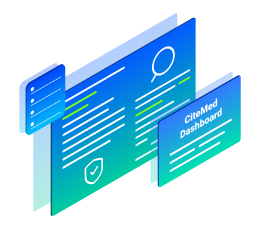
3. All Results Made Visible on Your Dashboard
Search results as well as our team’s reviews/classifications are immediately available for you to access on your CiteMed Dashboard.
4. Vigilance Report Document Is Delivered
On your chosen time interval, CIteMed delivers an Adverse Event Report to you via email and accessible directly on your CiteMed Dashboard.

All Your Vigilance Data In One Place

If You Are: