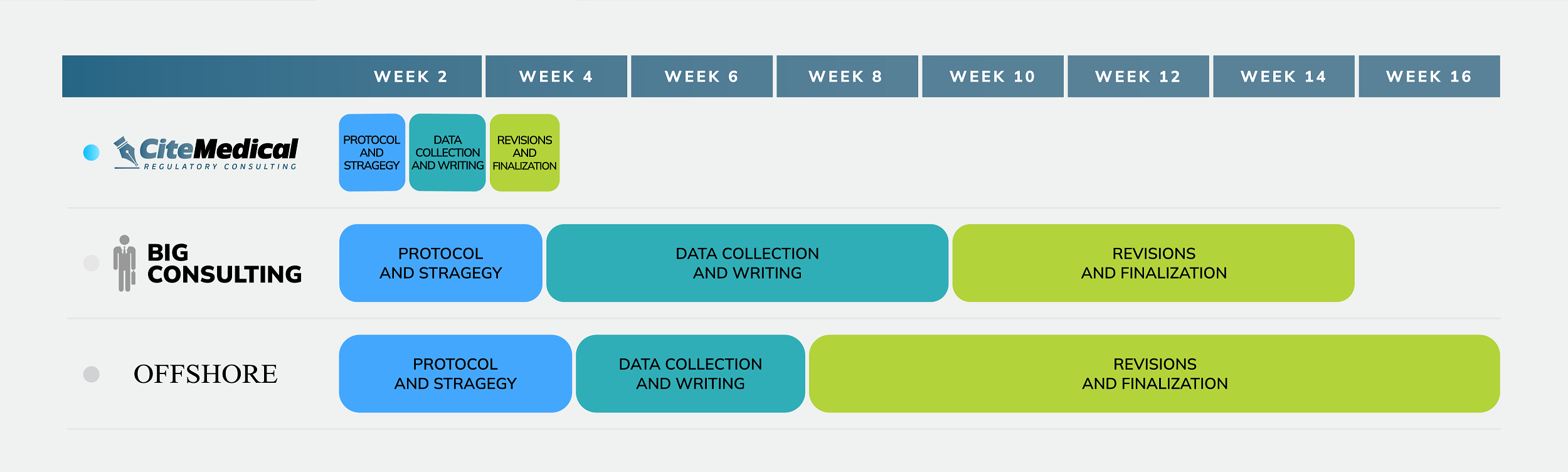
A PER Process
That Fits Your Team
Work With Us On Your Terms
Have some great writing staff already? Or limited budget? Plug directly into our process and use our expertise only when you need it.

Full PERs End to End
Our team of Notified Body Acceptable PER writers can deliver a complete document
ready for submission.

Use Our Templates
Draft your new MDR PER from our templates. Complete training annotations, and Notified Body insights included.
100% Success Rate
How We Do It:
We are one of the only writing teams out there confident enough to guarantee our work. But before you roll your eyes and mutter “Nothing’s guaranteed in Regulatory Affairs”… hear us out.
We only work with great products and even better teams: If your device or submission is headed for disaster because of unrealistic claims fraudulent clinical data, or if your team is full of jerks… we won’t be able to help.
You get support through the entire audit: We don’t just zip up a pile of documentation and send you on your way. All of our projects include free consulting hours to address Auditor feedback, and ensure your get through successfully.
See our track record for yourself: We have extensive lists of reference. Drop us a line and we’d be glad to put you in touch with clients who saw success using our team/methods.
Delivered On Your Timeline
An Audit Pro On Your Side
Notified Body Audits are tough to navigate on your own. Benefit from our teams countless submission successes and experience in drafting precise responses for all rounds of your Audit.

Dedicated Writing Team
Not all medical writers are created equal… which is why we hang on to our team of experience writing leads for dear life!
Our writing team is experienced, with cumulative 100s of successful documents authored and submissions navigated for clients.
Our writing team is qualified, all writers meet the Notified Body and EU Standards for a Clinical Reviewer/Author
Our writing team is diverse, dotting the globe geographically, and in terms of international regulatory requirements, you won’t find a more robust team.
IVDR Compliance challenges
Peace of Mind For Your Submission
Our team is built and trained to get your PER approved. Whether you need a complete service, or are just looking for some occasional doc review and literature support the CiteMed team is ready to jump in.
IVD services provided by Citemedical

Gap Assessments
- Device Classification as per IVDR (Classes A, B, C, D)
- Technical Documentation
- QMS development and implementation

Global Registrations
- UDI and Labelling Requirements
- Technical File Creation
- Submission and Review
- CE-Marking and International

Medical Writing
- Performance Evaluation Plan (PEP)
- Performance Evaluation Report (PER)
- Scientific Validity Report (SVR)
- Analytical Performance Report (APR)
- Clinical Performance Report (CPR)
- Post-Market Performance Follow-Up Plan (PMPF)
FEATURED IN

Are You Ready to Work With Us?

If You Are…
We might be a fit!


Notifed Body Approved Writers
Our writers have the chops to tackle any classification of device for IVDR, and the CVs to submit alongside your final document. Our team has cumulatively written well into the 100s of PERs on all device classifications.
Consistent Updates If You Need Them
Keep your Literature Review, and PER documents updated year after year without worry or hassle. We keep everything organized and audit ready.
Post Market and Vigilance Data Support
If you have a team and process in place for PMS, great! If you don’t, our comprehensive PMS packages are easily integrated into your submission.