Systematic Literature Reviews
The MDR forces manufacturers to set up processes for continuous literature reviews and reevaluation of the available clinical data. In May 2021, the European Medical Device Directive (93/42/EEC) was replaced with the Medical Device Regulation (MDR, 2017/745). Unlike MDD, the MDR considers clinical evaluation to take place during the entire lifecycle of medical devices, meaning clinical data must be updated continuously. Seeing as clinical data is usually sourced from clinical investigations, post-market surveillance, and literature reviews.
Literature reviews are fundamental to any scientific discipline, social sciences, medicine, or linguistics. Over the past 100 years, scientific documentation and sharing of new research have evolved into a labyrinthine field of publication databases, each housing thousands of articles, journals, research manuscripts, and conference summaries and abstracts. Yet, with all this knowledge at the tip of our fingers, how do we gather and select information for analyzing scientific queries, and how do we interpret the collected literature about our topic?
What is a Literature review?
Literature reviews are an analytical approach to investigating a specific topic or research question. For the scientist, they help gain insight into current knowledge, relevant theories and methods, and any gaps or missing exploration opportunities in the existing research. For the reader, they should help situate the research within the current body of knowledge and provide context to the exploration of the chosen research subject.
A literature review comprises four steps; investigation of published literature on the chosen topic; summarizing the chosen literature and synthesizing its relevance to the chosen topic; analyzing and critically evaluating the selected literature; and; presenting the literature in a structured way.
Literature reviews are performed for various scientific works, such as theses, dissertations, journal articles, books, conference manuscripts, scientific journalism, and patient brochures and instructions for use. Most importantly, literature reviews are vital in clinical evaluations of drugs and medical devices. The latter is the focus of this article.
In medical devices and clinical evaluations, literature reviews aim to provide the reader with context and background for the chosen treatment or disease or act as a general overview of a topic.
The systematic literature review is a type of literature review that aims to answer a specific clinical question. While literature reviews can take a few weeks to a few months by a single author, conducting a systematic review can take a team of up to five authors a year or more.
History of the systematic literature reviews and meta-analyses

The systematic literature review asks a specific clinical question about the effectiveness of an intervention or treatment. It seeks to answer it by critically summarizing and analyzing the current evidence for that intervention. It is considered the highest form of evidence-gathering for medical science. The systematic review process can be further elevated by using meta-analysis methods on data in the chosen literature, resulting in a completely objective evaluation of the research findings.
James Lind
James Lind, a Scottish doctor preoccupied with helping sailors in the royal navy avoid scurvy, was the author of the first-ever systematic literature review, in which he published a paper providing a clear and unbiased review of the existing evidence on scurvy. Although his article A treatise of the scurvy, published in 1753, was practically ignored, he started science on a path of systematic literature review that is still underway.
Archie Cochrane
In 1972, Archie Cochrane wrote:
“It is surely a great criticism of our profession that we have not organized a critical summary, by specialty or subspecialty, adapted periodically, of all relevant randomized controlled trials.”
Gene Glass and Mary Lee Smith
Research synthesis and analysis emerged formally in 1975 when Gene Glass and Mary Lee Smith coined the term meta-analysis in their research paper Meta-analysis of psychotherapy outcome studies1.
During the rise of evidence-based medicine in the late 1970s and early 1980s, systematic research analysis and synthesis were applied to medicine and health. For example, researchers in Oxford began a program of systematic literature reviews on the effectiveness of health interventions, thus paving the way for evidence-based medicine and committing to the principles of accumulative scientific knowledge.
Characteristics of a Systematic Literature Review protocols

Systematic reviews are frequently used in evidence-based healthcare, public health interventions, and evidence-based policy and practice. They are designed to provide a comprehensive and complete summary of the current literature on a specific topic and to minimize bias in the analysis and interpretation of the collected research.
Many systematic literature reviews include meta-analysis, which applies statistical analysis to the available research. In contrast, others focus on the qualitative synthesis of data or mixed-method reviews that have both.
The systematic literature review should display the following characteristics:
Clearly defined and specific research questions and objectives
- Pre-defined inclusion and exclusion criteria for choosing relevant literature
- Pre-defined and systematic search strategy, including search terms and keywords
- Defined eligibility criteria to be applied to all the Specified used in the review
- A systematic and clear evaluation of the quality of the literature included
- Identification of excluded sources and justifications for exclusion
- Analysis of the gathered data and information
- References to incoherences, errors, and limitations in and of the chosen literature
What does PRISMA stand for?
The PRISMA framework (Preferred Rating for Systematic Reviews and Meta-analyses) was developed to ensure a standardized way of conducting a systematic review to ensure transparency and completeness. The framework is now required by more than 170 medical journals worldwide. In addition, it has been extended to support specific review types or aspects of the review process, for example, PRISMA-P for review protocols and PRISMA-ScR for scoping review.
What are the other tools used for Systematic Literature Review?
Similarly, the ENTREQ (Enhancing Transparency Reporting the Synthesis of Qualitative Research) guidelines exist for quantitative reviews, RAMESES (Realist and Meta-narrative Evidence Syntheses) for meta-narrative and realist reviews, and eMERGe (Improving Reporting of Meta-Ethnography) for meta-ethnography reviews.
How to conduct systematic literature reviews and meta-analyses?

Defining your study objective/research question
The definition of the research question and/or study objectives is the most important part of the systematic review. Without a clearly defined research question and quantifiable endpoints, the rest of the review can lack focus and accuracy, ultimately ending up with conclusions and weak and unfocused literature interpretations. Care should be taken to avoid research questions that are too wide or too narrow.
For medical interventions and treatments, the PICO method is frequently used. The PICO framework helps develop search strategies focused on the patient, population, or problem (P); intervention (I); comparison, control, or comparator (C); and; outcome (O).
Lietrature Search Protocol
A clearly defined set of criteria must be set up to include or exclude research on the topic, and these criteria are included in the search protocol. The search protocol is your master document that guarantees transparency, repeatability, and audibility for your review.
One of the most critical aspects of the systematic review is that it can be objectively evaluated for the accuracy of methods and search criteria, and the search protocol ensures that. The search protocol should be carefully planned according to the research question and explicitly documented before the review starts. It supports the review team, ensuring consistency and integrity in all the carried searches.
Once the review is complete, you should be able to identify every paper you read and every piece of information you searched, critiqued, and defined. While doing all this, it is important to include everything – from the terms you used to search, strategies incorporated, and limitations you encountered along the way.
Literature Search Databases
Literature for clinical evaluations can be found in scientific journals, academic dissertations, books, bibliographic databases, and online databases. Most systematic reviews are based on 5-7 online publication sites, such as PubMed, Cochrane, and Embase (see below for more information), where publications are typically journal articles or conference abstracts. Literature can also be found in “gray” sources, such as dissertations, theses, fact sheets, government reports, and pre-prints of articles. Every source should be included, analyzed, synthesized, and acknowledged in a comprehensive literature review. It is also important to review multiple sources to avoid publication bias in the data interpretation. It is important to apply appropriate use of terminology when searching for literature. Unless a commonly accepted group of terminologies are used, a gap remains in data encryption – be it manual or automated. It is easy to find databases and thesauri highlighting common terminologies used in various fields. Furthermore, alternative spellings and similar conceptual roots should also be considered to ensure all relevant publications are included and evaluated. Boolean expressions can widen, focus, and improve search scope in almost all databases and should also be considered.
Data analysis and synthesis

Once you’ve done your searches, the eligibility criteria from your search protocol come into effect, and the methodological quality of your evaluation criteria is tested. Your data extraction from the literature should be performed precisely as established in the search protocol. In addition, it must be recorded so that it can be replicated faithfully by others in the future.
After selecting your data and literature, you are ready to start analyzing it. The more data is included in your review literature, the better and more unbiased your results will be. If appropriate and established in your search protocol, you can use meta-analysis (i.e., analyzing data from multiple sources through statistical methods) to analyze your data or other analytical tools, such as qualitative meta-synthesis, which is the synthesis of data from qualitative studies.
Data presentation
Presenting your findings can be challenging, as the outcome should be clearly structured, easily understood, and advanced in a natural and chronological order. Most systematic literature reviews are presented in article form and published in scientific journals and generally present the same structure:
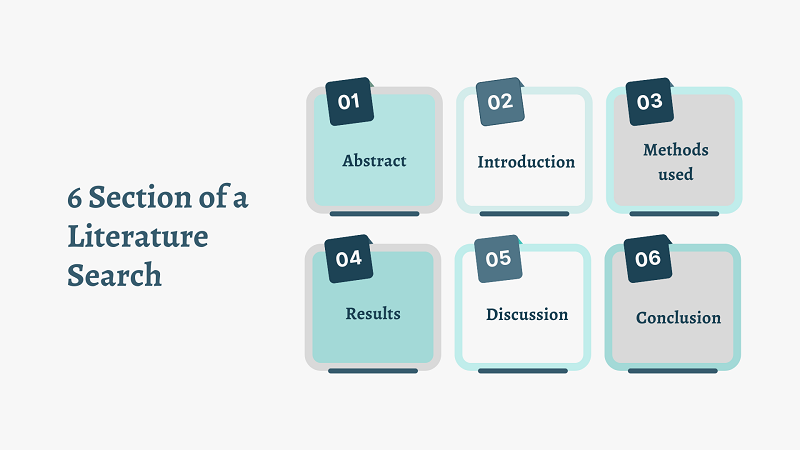
These sections are followed by acknowledgments, references, and tables and figures (the latter may also be included in the general text, depending on the journal).
Resources
Cochrane

Cochrane is the largest, and arguably one of the most important, international organizations in medicine and health today. Cochrane consists of more than 37,000 specialists in healthcare fields who systematically review randomized trials. Cochrane reviews are published in the Cochrane Library, in the Cochrane Database of Systematic Reviews section, and are a valuable resource for anyone looking to conduct a systematic literature review.
PubMed

PubMed is a free publication resource, housing more than 32 million citations and abstracts of biomedical literature. Although PubMed does not provide full-text citations, they link their citations to the full text, often through the publisher’s website. The largest component of Pubmed is MEDLINE, which consists most significant actions from biomedical publications through the National Library of Medicine.
Embase

Embase is another comprehensive medical literature database, similar to Pubmed, where you can search for full-text content or abstracts. Embase is focused on supporting pharmacovigilance and regulatory authorities and includes biomedical and pharmacological publications from 1947.
PubMed and Embase are powerful search engines for retrieving biomedical and life science literature. However, both sites require a thorough knowledge of the optimization of their search engines, the use of BOOLEAN operators, and the indexation systems of Medical Subject Headings, MeSH.
Systematic literature reviews and the EU MDR
In May 2021, the European Medical Device Directive (93/42/EEC) was replaced with the Medical Device Regulation (MDR, 2017/745). Unlike MDD, the MDR considers clinical evaluation to take place during the entire lifecycle of medical devices, meaning clinical data must be updated continuously.
Seeing as clinical data is usually sourced from clinical investigations, post-market surveillance, and literature reviews, the MDR forces manufacturers to set up processes for continuous literature reviews and reevaluation of the available clinical data.
Several sections of the MDR propose using literature to support and source clinical data, such as for demonstrating equivalence, post-market clinical follow-up, clinical investigation plans, and investigators’ brochures. As such, systematic literature reviews are an invaluable part of sourcing clinical data for each clinical section in the MDR.
1) Smith, Mary & Glass, Gene. (1977). Meta-Analysis of Psychotherapy Outcome Studies. The American psychologist. 32. 752-60. 10.1037//0003-066X.32.9.752.
