Clinical Evaluation Report (CER)
A clinical evaluation report is the report a manufacturer is expected to submit to the European regulatory authority to provide details obtained from the documentation of the clinical evaluation process of a specific medical device.
It provides the regulatory authority with a clear image of the medical device’s performance in a clinical setting. Hence the report must be accurate and thorough.
This article will provide definitions, parameters, scopes, and associated document links to create a clinical evaluation report (CER).
Clinical evaluation

According to MDR article 2 (44), clinical evaluation can be defined as
“a systematic and planned process to continuously generate, collect, analyse and assess the clinical data pertaining to a device in order to verify the safety and performance, including clinical benefits, of the device when used as intended by the manufacturer.”
The clinical evaluation report (CER) reflects the defined and methodologically sound procedure of clinical evaluation based on which a device can be declared as conforming to the MDR requirements. As such, the clinical evaluation process should:
- Be justifiable in its level of clinical evidence based on the device’s intended purpose.
- Be based on the relevant, current scientific literature related to the device’s intended purpose, design, safety, and performance.
- Have an assessment of the results of the related clinical investigations, which should be performed according to Articles 62 to 80.
- List currently available alternative treatment options.
- Be based on the device’s technical, biological, and clinical characteristics.
Manufacturers are expected to plan, execute and document the clinical evaluation and submit documents on each step for appropriate devices.
For products with no clear intended purpose, manufacturers must show the product’s clinical benefits. The clinical evaluation will conduct the relevant tests to demonstrate this claim.
Clinical evaluation report

According to the Eu regulation 2017/745, the clinical evaluation reports and associated clinical evidence make up the clinical evaluation report. The CER helps to prove the conformity of the device.
The report will contain the clinical evidence along with non-clinical data, which are gained through non-clinical tests and any relevant document. Therefore, it is imperative that all clinically significant results, both favorable and unfavorable, are included in the report.
The clinical evaluation report (CER) is expected to be updated as necessary.
Clinical evaluation plan

According to the Part A of Annex XIV of MDR, the medical device manufacturer shall create a clinical evaluation plan that will include the following:
- Clinical data-based parameters of general safety and performance requirements
- Intended purpose and its specific identification.
- The intended target group of the device with concise indication and contraindication.
- Identification of the methods used in the examination of clinical safety.
- List of parameters with details that were used to determine the benefit-risk ratio based on the indication by comparing the device to state of the art.
- Explicit declaration on how the benefit ratio of various components fits in aspect to human tissue, pharmaceuticals, and non-viable tissue.
- A clinical development plan contains developments from exploratory to confirmatory investigations, with parameters and details of potential acceptance criteria.
Clinical investigation
It is defined as
“any systematic investigation involving one or more human subjects, undertaken to assess the safety or performance of a device.”
Clinical investigation is different from clinical evaluation. The investigation acts as a tool in the evaluation process.
Clinical investigation plan

It is defined as
“a document that describes the rationale, objectives, design, methodology, monitoring, statistical considerations, organization, and conduct of a clinical investigation.”
This includes a detailed plan of how the clinical investigation will take place.
Clinical data
Not all data can qualify as clinical data. MDR has a specific definition and criteria for clinical data. It can be defined as “information concerning safety or performance that is generated from the use of a device.”
Clinical data can be gained from
- “Clinical investigation(s) of the device concerned.
- Clinical investigation(s) or other studies reported in the scientific literature of a device for which equivalence can be demonstrated to the device in question.
- Reports published in the peer-reviewed scientific literature on other clinical experiences of either the device in question or a device for which equivalence to the device in question can be demonstrated.
- Clinically relevant information comes from post-market surveillance, particularly the post-market clinical follow-up.”
Clinical evidence
Clinical evidence is needed to prove conformance. It is defined as
“clinical data and clinical evaluation results pertaining to a device of a sufficient amount and quality to allow a qualified assessment of whether the device is safe and achieves the intended clinical benefit(s), when used as intended by the manufacturer.”
Clinical performance
Clinical performance is required to be up to a specific standard. It is defined as
“the ability of a device, resulting from any direct or indirect medical effects which stem from its technical or functional characteristics, including diagnostic characteristics, to achieve its intended purpose as claimed by the manufacturer, thereby leading to a clinical benefit for patients, when used as intended by the manufacturer”. The performance of A medical device must be defendable for it to get a CE marking.
Intended purpose
The intended purpose also acts to defend the device’s use. It is defined as
“the use for which a device is intended according to the data supplied by the manufacturer on the label, in the instructions for use or in promotional or sales materials or statements and as specified by the manufacturer in the clinical evaluation.” A device’s clinical effect must match the intended purpose.
State of the art
It is the standard to which a device will be compared. It can be defined as
“developed stage of current technical capability and/or accepted clinical practice in regard to products, processes and patient management, based on the relevant consolidated findings of science, technology and experience.”
Steps of clinical evaluation and data collection for clinical evaluation report
According to the MEDDEV 2.7/1 revision 4, which is still relevant on topics where MDR doesn’t give otherwise instructions, the scope and suggested steps of clinical evaluation and subsequent clinical evaluation report are provided.
The steps, in short, are:
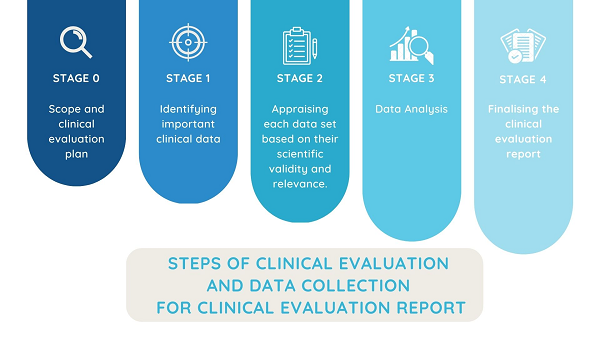
Summary of the clinical evaluation findings and drawing relationships between the found literature and parameters of the technical documents.
Further reading
- Clinical Evaluation: A Guide For Manufacturers And Notified Bodies Under Directives 93/42/EEC and 90/385/EEC
- Regulation (EU) 2017/745 of The European Parliament and of the Council of 5 April 2017